SUMMARY
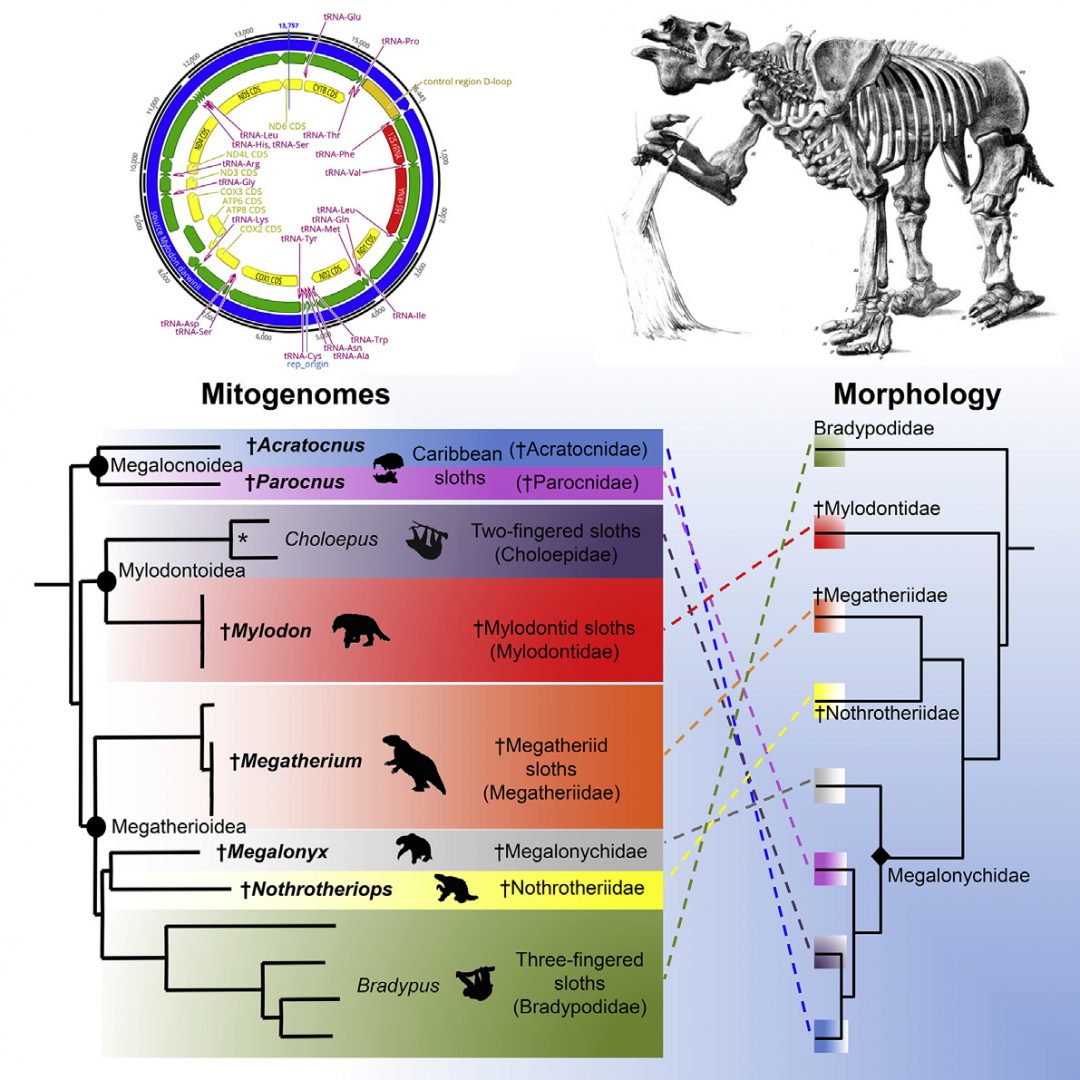
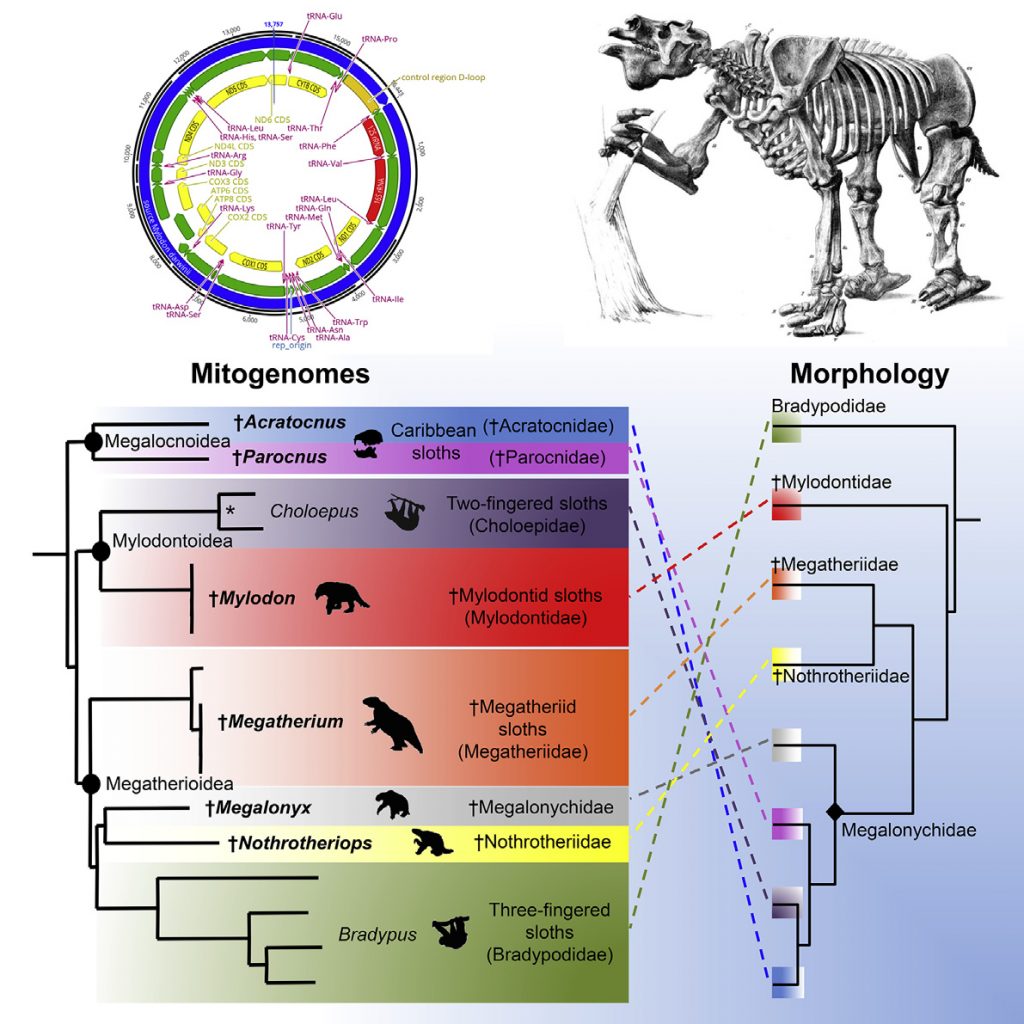
Living sloths represent two distinct lineages of small-sized mammals that independently evolved arboreal-ity from terrestrial ancestors. The six extant species are the survivors of an evolutionary radiation marked by the extinction of large terrestrial forms at the end of the Quaternary. Until now, sloth evolutionary his-tory has mainly been reconstructed from phylogenetic analyses of morphological characters. Here, we used ancient DNA methods to successfully sequence 10 extinct sloth mitogenomes encompassing all major lineages. This includes the iconic continental ground sloths Megatherium, Megalonyx, Mylodon, and Nothrotheriops and the smaller endemic Carib-bean sloths Parocnus and Acratocnus. Phylogenetic analyses identify eight distinct lineages grouped in three well-supported clades, whose interrelationships are markedly incongruent with the currently accepted morphological topology. We show that recently extinct Caribbean sloths have a single origin but comprise two highly divergent lineages that are not directly related to living two-fingered sloths, which instead group with Mylodon. Moreover, living three-fingered sloths do not represent the sister group to all other sloths but are nested within a clade of extinct ground sloths including Megatherium, Megalonyx, and Nothrotheriops. Molecular dating also reveals
that the eight newly recognized sloth families all orig-inated between 36 and 28 million years ago (mya). The early divergence of recently extinct Caribbean sloths around 35 mya is consistent with the debated GAARlandia hypothesis postulating the existence at that time of a biogeographic connection between northern South America and the Greater Antilles. This new molecular phylogeny has major implications for reinterpreting sloth morphological evolution, biogeography, and diversification history.
INTRODUCTION
Sloths (Xenarthra; Folivora) are represented today by six living species, distributed in tropical forests throughout the Neotropics and conventionally placed in two genera: Choloepus, the two-fingered sloths (two species), and Bradypus, the three-fingered sloths (four species). Tree sloths typically weigh 4–8 kg and are strictly arboreal. However, the living species represent only a small fraction of the past Cenozoic diversity of sloths. More than 100 genera of sloths have been systematically described, including the large-bodied species of the Pliocene and Pleisto-cene popularly known as ground sloths of the Ice Age. This includes the giant ground sloth (Megatherium americanum) with an estimated body mass of more than 4,000 kg and Darwin’s ground sloth (Mylodon darwinii), named for Charles Darwin, who collected its first fossil remains. Like their closest xenarthran relatives (anteaters and armadillos), sloths originated in South America and successfully invaded Central and North America prior to the completion of the Isthmus of Panama [1]. Pleistocene North American representative taxa include the Shasta ground sloth (Nothrotheriops shastensis) and Jefferson’s ground sloth (Megalonyx jeffersonii), whose range extended up to Alaska. Late Quaternary ground sloths went extinct ?10,000 years before present (yrbp) as part of the megafaunal extinction that occurred at the end of the latest glaciation [2]. However, sloths also reached a number of Caribbean islands, giving rise to an endemic radiation best known from Quaternary taxa (Megaloc-nus, Neocnus, Acratocnus, and Parocnus)[3] that became extinct only shortly after the appearance of humans in the Greater Antilles ?4,400 yrbp [4]. When and how sloths colonized the West Indies is still disputed. The oldest accepted fossil evidence dates from the early Miocene of Cuba [5], although discoveries in Puerto Rico [6, 7] demonstrate that terrestrial mammals, possibly including sloths, were already in the Greater Antilles by the early Oligocene. These findings would be consistent with the debated GAARlandia (GAAR: Greater Antilles + Aves Ridge) paleobiogeo-graphic hypothesis postulating the existence of a land bridge via the Aves Ridge that would have briefly emerged between 35 and 33 million years ago (mya) and connected northern South America to the Greater Antilles [6].
Until recently, the phylogenetic relationships of sloths were almost exclusively investigated from analyses of morphological data. Cladistic analyses using maximum parsimony [8–11]and Bayesian reconstructions [12] based predominantly on cranio-dental characters have consistently recovered topologies defining five major sloth lineages, currently recognized as families. In these phylogenetic reconstructions, modern three-fingered sloths al-ways appear as the sister group of all other sloths and are consid-ered to have retained a number of ancestral characters [8]. Extant two-fingered sloths are also consistently found close to or nested within Caribbean sloths as the sister-group of either Acratocnus [3] orNeocnus [8, 12] and are classified within Megalonychidae, together with other extinct sloths related to Megalonyx.Itisnote-worthy, however, that there is currently no fossil that could be convincingly assigned to the two independent lineages that led to extant tree sloths [13]
The vast majority of Quaternary sloth taxa became extinct so recently that numerous remains in the form of bones, teeth, frag-ments of skin with hair and osteoderms, claws with their kerati-nous sheaths, and paleofeces are still well preserved. The amount of subfossil material available makes sloths an ideal group to leverage the power of ancient DNA to decipher their ra-diation. In a pioneering study, Ho¨ ss et al. [14] tested 45 samples from diverse sloth taxa, but only two specimens of Darwin’s ground sloth (Mylodon darwinii) from Mylodon Cave (Chile) yielded short mitochondrial ribosomal gene fragments. Recently, a bone from the same cave with high endogenous DNA content allowed assembly of a high-quality complete mitogenome for Mylodon darwinii using shotgun sequencing [15]. Exceptional preservation of paleofecal material of the extinct Shasta ground sloth (Nothrotheriops shastensis) from the Gypsum Cave (Nevada) enabled characterization of its diet by ancient DNA barcoding of plant remains [16, 17]. Paleofeces from this cave also yielded short PCR-amplified mitochondrial [18] and nu-clear [19] sequences allowing investigation of the phylogenetic affinities among extinct and extant sloths. Nowadays, DNA cap-ture-based targeted enrichment is emerging as the method of 2032 Current Biology 29, 2031–2042, June 17, 2019
choice in ancient DNA studies. It has recently been used to reconstruct partial mitogenomes for Nothrotheriops shastensis and Mylodon darwinii [20]. Moreover, it has been demonstrated that baits designed from ancestral sequences reconstructed from extant xenarthran mitogenomes can improve capture suc-cess from species for which there is no closely related extant taxa such as the extinct glyptodont Doedicurus [21].
Both molecular [14, 15, 18–20] and morphological [8, 9, 12] phylogenetic studies have supported the diphyletic origin of the two living sloth genera, implying an independent evolution of arboreality from terrestrial ancestors. However, molecular studies are actually in conflict with morphological inferences regarding the precise phylogenetic positions of extant sloths in strongly supporting a close relationship between Choloepus and Mylodon [14, 15, 18, 20] and firmly grouping Bradypus with Nothrotheriops [18–20]. In order to understand the causes of this incongruence, we used ancient DNA techniques to sequence the mitogenomes of 10 extinct Quaternary sloths. Phylogenetic analyses of these new mitogenomic data support a topology that is markedly incongruent with the currently accepted morphological framework. Our results have major im-plications for interpreting sloth morphological evolution and should stimulate a complete rethinking of our current under-standing of the evolutionary history of this group.